What Stays Constant in a Dilution
Concentration is the removal of solvent which increases the concentration of the solute in the solution. Serial dilutions involve diluting a stock or standard solution multiple times in a row.
3 2 3 Concentration Chemistry Libretexts
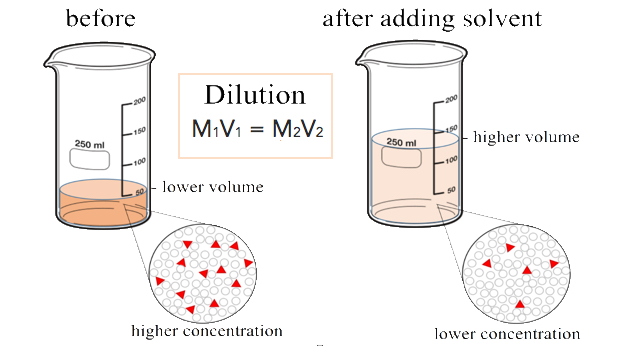
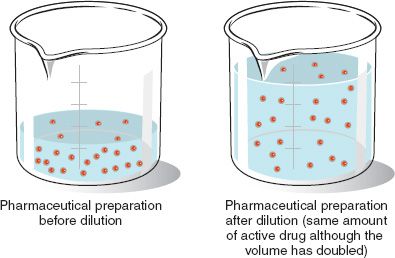
In both dilution and concentration the amount of solute stays the same.
. What stays constant in a dilution. See the answer See the answer See the answer done loading. And thus concentration of solute WILL DECREASEhowever the amount of dissolved substance will remain constant.
In both the dilution and concentration processes the amount of solute stays the same. One is a dilution and the other is a ratio. Concentration is the removal of solvent which increases the concentration of the solute in the solution.
What is a dilution. Select the correct answer below. In the scientific literature if you see 12 it means to add.
11 cells 40mL 0275 cellsmL. To bring this 2 ml sample up to a total volume of 10 ml you must add 10 ml 2 ml 8 ml diluent. It will have no effect on the moles of solute present in the initial solution.
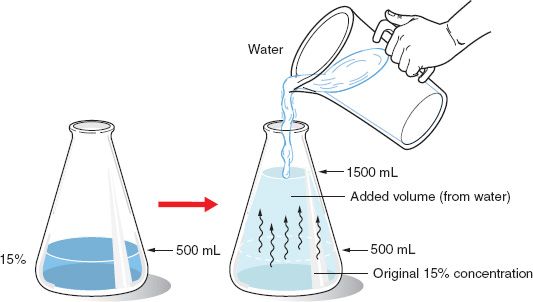
Dilution is the process of decreasing the concentration of a solute in a solution usually simply by mixing with more solvent like adding more water to the solution. So the moles of the solute remain constant before and after dilution. Dilution is the addition of solvent which decreases the concentration of the solute in the solution.
Initially we have a concentration of Moles of solute Volume of solution. The cell density of 3 is 044 cells mL. In both dilution and concentration the amount of solute stays the same.
Do not confuse the two uses of the word concentration here In both dilution and concentration the amount of solute stays the same. 15 dilution 15 dilution 1 part sample and 4 parts diluent in a total of 5 parts. In any dilution the number of moles of solute stays the same.
This problem has been solved. To dilute a solution add more solvent without the addition of more solute. We can solve for the number of moles of solute.
For example suppose you wanted to prepare 100mL of a solution of NaOH at 0lmolL-1. Using where is the molar conductivity at concentration c and is the limiting. It means to dilute something in half.
This means even though the equilibrium constant is much smaller than one you still can have reactants and products at the same concentrations. Using the bench reagent commonly containing 20molL- 20 M the dilution factor is 01 divide 20 005 120 a twenty-fold dilution. Note that volume percent is relative to the volume of the solution not the volume of solvent.
Both CH 4 and O 2 conversions fall with increasing dilution. For those type of reactions dilution favors the products. In a dilution only the solvent water is added but not solute.
For example wine is about 12 vv ethanol. Typically the dilution factor remains constant for each dilution resulting in an exponential decrease in concentration. Dilution refers to a drop in the pH of a chemical which can be a gas vapour or solution.
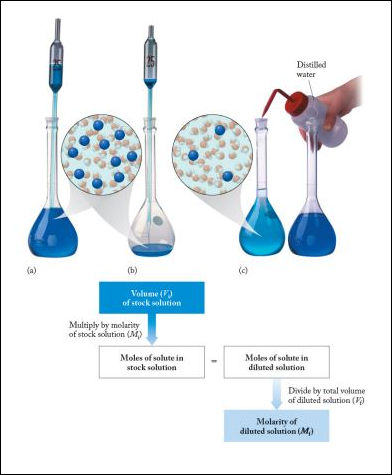
The dilution factor can be used to calculate the volumes and stock and diluent required in a particular instance. This gives us a way to calculate what the new solution volume must be for the desired concentration of solute. For example a ten-fold serial dilution could result in the following concentrations.
A 1 to 2 dilution should be written as ½. If you need 10 ml final volume then you need 15 of 10 ml 2 ml sample. These two forms are actually not equal despite the fact that they are used interchangeably in the laboratory.
Wilhelm Ostwalds dilution law is a relationship proposed in 1888 between the dissociation constant K d and the degree of dissociation α of a weak electrolyteThe law takes the form Where the square brackets denote concentration and c 0 is the total concentration of electrolyte. This gives us a way to calculate what the new solution volume must be for the desired concentration of solute. Show transcribed image text.
Dilution is the addition of solvent which decreases the concentration of the solute in the solution. From the definition of molarity molarity moles of solute liters of solution. The same direct relationship applies to.
044 16 0275 cellsmL. Now dilution will increase the volume. The nitrogen dilution was varied between 10 and 40 at a CH 4 O 2 ratio of 17 and a constant GHSV of 18 x 10 5 hr- 1.
The resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. You are simply increasing the amount of solvent in the solution. It involves the process of decreasing the concentration of a solute in the solution normally by mixing with the solvent.
To dilute a solution means to add more solvent without the addition of more solute. CH 4 conversion goes from 64 at a N 2 dilution of 10 to just over 30 at 40 dilution. Moles litresmoleslitres volume molarity Vc.
What stays constant in a dilution. As a result this gives us a way to calculate what the new solution volume must be to get the desired concentration of the solute. But many times it will be written as 12.
From the definition of the molarity we know molarity. We can solve for the number of moles of solute. The process of addition of water to a stock solution to obtain a solution of desired molarity is called dilution.
1 M 01 M 001 M 0001 M and so on. You use the formula V1c1V2c2. Vv volume of solute volume of solution x 100.
Which of the following stays constant when diluting a solution. In fact if K 10 5 for this reaction and all concentrations are 10 5 M the reaction is at equilibrium. From the definition of molarity molarity moles of solute liters of solution.
We divide the cell density by the dilution factor and we get. Oxygen conversion falls from 100 at 10 dilution to less than 80 at 40. Do not confuse the two uses of the word concentration here In both dilution and concentration the amount of solute stays the same.
Volume molarity O concentration amount of solute. Explain why the equation M1 V1M2 V2works for dilution problems. Mix the resulting solution thoroughly to ensure that all parts of the solution are even.
This means there is 12 ml ethanol for every 100 ml of wine. The dilution factor in this step is 40mL 25mL 16. Explain why the equation M1 V1M2 V2works for dilution problems.
Same thing for the dilution from 3 to 5.

Dilution And Concentration Basicmedical Key

Comments
Post a Comment